Research Article
Comparative Analysis of Antioxidant Activity of Milk Phospholipids from Different Milk Formulations
- G. Shivaani
Corresponding author: G. Shivaani, Master of Science in Biochemistry, from the Department of Biochemistry and Nutrition, Bhavan's Vivekananda College, Secunderabad, India.
Volume: 2
Issue: 2
Article Information
Article Type : Research Article
Citation : G. Shivaani. Comparative Analysis of Antioxidant Activity of Milk Phospholipids from Different Milk Formulations. Journal of Medicine Care and Health Review 2(2). https://doi.org/10.61615/JMCHR/2025/APRIL027140430
Copyright: © 2025 G. Shivaani. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
DOI: https://doi.org/10.61615/JMCHR/2025/APRIL027140430
Publication History
Received Date
04 Apr ,2025
Accepted Date
22 May ,2025
Published Date
01 May ,2025
Abstract
Milk phospholipids are a complex of lipids that are essential components of the cell membrane and have been shown to possess antioxidant properties. Studies have shown that milk phospholipids can scavenge free radicals, inhibit lipid peroxidation, and increase antioxidant enzyme activity, thereby reducing oxidative stress. Additionally, they have been shown to have anti-inflammatory effects, modulate immune function, and improve lipid metabolism. Research has also investigated the potential health benefits of milk phospholipids, including their role in reducing the risk of cardiovascular disease, supporting brain function, and promoting healthy aging. Clinical studies have shown that supplementation with milk phospholipids can improve cognitive function, reduce inflammation, and improve lipid profiles in individuals with metabolic disorders. Overall, milk phospholipids appear to have significant antioxidant properties and potential applications in promoting human health. The present study aims to compare the antioxidant activity of different milk formulations given as supplements to children and elderly people. The methodology involved the extraction and isolation of milk phospholipids from commercially available milk formulations and analyzing their antioxidant potential by assaying for hydrogen peroxide scavenging activity and free radical scavenging activity by FRAP assay. According to the results of analyzing various milk powders, all of the phospholipids were found in Glucipro and Nestle. In contrast, Similac had high scavenging activity, then as the extract's concentration increased, Amulya's milk powder demonstrated greater reducing power. Further research is needed to fully understand the mechanisms by which milk phospholipids exert their biological effects and to determine the optimal doses and formulations for specific health benefits.
Keywords: Milk phospholipids, Antioxidant activity, FRAP assay, Hydrogen peroxide scavenging, Lipid extraction, Thin Layer Chromatography (TLC), Comparative analysis, Dairy lipids.
►Comparative Analysis of Antioxidant Activity of Milk Phospholipids from Different Milk Formulations
G. Shivaani1*
1Master of Science in Biochemistry, from the Department of Biochemistry and Nutrition, Bhavan's Vivekananda College, Secunderabad, India.
Introduction
Milk phospholipids have been used as functional ingredients in foods. Glycerophospholipids and sphingolipids are quantitatively the most important phospholipids (PLs) in milk, and their amphiphilic properties derive from the presence of both a hydrophobic tail and a hydrophilic head [1]. Sphingomyelin is the predominant species of sphingolipids, and they are mostly of phosphatidylcholine, phosphatidylethanolamine, phosphatidylglycerol, and phosphatidylserine. They belong to the class of polar lipids and are defined as “lipids containing phosphorus.” They are the main constituents of the milk fat globule membrane (MFGM), which encircles the lipid droplets secreted by the mammary gland cells [2].
Phospholipids have been used to prepare antioxidant phytosomes and to modulate the release of antioxidants from phospholipid vesicles. Phospholipids (PLs) are found as lipid bilayers in all plant and animal cell membranes [3]. The term PLs refers to a class of complex polar lipids containing a phosphate group and two fatty acids esterified to a glycerol backbone. PLs derived from milk are primarily rich in sphingomyelin (SM) and phosphatidylserine (PS), two of the most highly bioactive PLs [4]. Milk phospholipids have antioxidant properties that can help reduce oxidative stress in the body. They have also been found to exhibit neuroprotective effects, improve memory and resistance to stress, and reduce the risk of certain diseases [5]. There is strong evidence that PLs have positive impacts on health, including control of inflammatory responses, chemopreventive and chemotherapeutic efficacy against various cancers, and suppression of cholesterol absorption. phospholipids include soya, rapeseed, sunflower, chicken eggs, and fish eggs [6].
It has been determined that functional foods like milk and its products have antioxidant properties that may improve human health, immunity, and nutritional value, prevent, or treat health threats, aid in the fight against some diseases like diabetes and cancer, lower blood pressure and cholesterol-related issues, as well as ameliorate cardiovascular disorders, lower risk of chronic diseases, and lessen allergic reactions Additionally, milk phospholipids can induce antioxidant genes and have barrier-protective properties. Mammalian milk, including cow, buffalo, camel, goat, and ewe milk, is a prominent source of milk phospholipids. Skimmed milk, Buttermilk, and butter serum are high-phospholipid dairy sources. Other sources of industrially produced phospholipids include soya, rapeseed, sunflower, chicken eggs, and fish eggs [7].
Designer foods like milk powders include antioxidants, which can be utilized to treat lifestyle problems. Milk also contains appreciable amounts of equol, a polyphenolic metabolite of daidzein, and the antioxidant activity of this equol is scientifically well-known. Superoxide radicals (O2−), hydroxyl radicals, and peroxide radicals can be inhibited by the antioxidant systems of milk [8]. Here are a few examples of relevant research on the antioxidant properties of phospholipids and their relevance in health or industry: From most of the studies, it became evident that dietary PLs have a positive impact on several diseases, apparently without severe side effects. Furthermore, they were shown to reduce the side effects of some drugs. Both effects can partially be explained by the fact that PL is highly effective in delivering its fatty acid (FA) residues for incorporation into the membranes of cells involved in different diseases, e.g., immune or cancer cells [9]. In a study published in the journal Nutrients in 2020, researchers investigated the antioxidant properties of phospholipids derived from krill oil. They found that krill oil phospholipids had a stronger antioxidant effect than other types of omega-3 fatty acids, which are also known for their antioxidant properties. The researchers suggested that krill oil phospholipids may have the potential as a dietary supplement to support an antioxidant defense and reduce oxidative stress [10]. In a review article published in the journal Advances in Nutrition in 2016, researchers summarized the current evidence on the health benefits of phospholipids, including their antioxidant properties. The authors noted that phospholipids have been shown toimprove lipid metabolism, reduce inflammation, and support brain function, among other brain function, among other potential benefits. They suggested that further research is needed to fully understand the mechanisms by which phospholipids exert their health effects [11]. Recent research has focused on nutritional interventions to modify behaviour and reduce the deleterious consequences of both stress and aging. In this context, emerging evidence indicates that phospholipids, a specific type of fat, can improve a variety of cognitive processes in both animals and humans. The mechanisms underlying these positive effects are actively being investigated. They summarised the preclinical and clinical studies available on phospholipid-based strategies for improved brain health across the lifespan [12]. The samples that were taken are Amulya milk powder, Nestle milk powder, Glucipromilk powder (diabetic), Dairy Whitener (Ensure), Ensure milk powder, and Nestle milk powder.
Materials
The samples that were taken are Amulya milk powder, Nestle milk powder, Glucipromilk powder (diabetic), dairy whitener, Ensure milk powder, and Nestle milk powder.
Methanol, chloroform, NaCl, H2O2, Glass column, Silica gel G for TLC, Silica gel, Phosphate Buffer, Potassium ferricyanide, Trichloroacetic acid, and Ferric chloride (freshly prepared).
Methods
- Isolation of Phospholipids
A modified Folch technique was used to extract the phospholipids from milk. (Nollet & Toldrá, 2015). Milk powder (20 g) was first dissolved in 450 mL methanolic chloroform (1:2, v/v). After 20 min of agitation, 160 mL of saline aqueous solution (0.74 g NaCl in 100 mL solution) was added to precipitate the casein proteins. Following another 20 min of mixing, the mixture was centrifuged at 4000g for 30 min at 4 °C. Subsequently, the aqueous supernatant was withdrawn with a 5 mL pipette, while the solidified layer was removed with a scraper. Milk lipids in the chloroform layer (denser bottom layer) were then air-dried.
- Separation of Chloroform Layer
The chloroform layer was separated from the mixture by using a separating funnel, and the contents were placed on a hot plate at 50°C, and The yield was calculated gravimetrically.
- Separation And Identification of Various Phospholipids by Column Chromatography
The silica gel (5g) was washed with double-distilled water to remove fine particles and activated at 125°C for 14h. Suspended the activated silica gel (5g) in chloroform(10 mL) and carefully poured the slurry into the glass column with having glass wool plug at its bottom. Allowed absorbent to settle and added more slurry till a column with a bed height of 6.8cm is obtained. Washed the packed column with three-column volumes of chloroform. 1 mL of lipid extract was added to the silica gel column and eluted with 10 mL of chloroform at a constant flow rate of 1 mL/min. The neutral lipids, i.e., triglycerides, diacylglycerols, sterols, and sterol esters, were eluted during this step. The polar lipids, which remained adsorbed in the column during the previous step, were then eluted with 10 mL of chloroform. These fractions contained glycolipids and sterol glycolipids. The residual glycolipids and all the phospholipids were finally eluted from the column with 10 mL of methanol. Collected fractions of 1ml each and summed them together to make it 5ml and examined for the presence of galactose and phosphorus. Later, it was placed in an Eppendorf with a lid open to allow methanol to evaporate at room temperature, and lipid fractions were found at the bottom.
- Thin-Layer Chromatography of Lipids
In thin-layer chromatography solvent made up of toluene, ethyl acetate, and ethanol (2:1:1), was used to identify galactolipids and phospholipids. The slurry was prepared with silica gel G and distilled water, the ratio being 1:2, which was plated after mixing the thick paste. The plates were then activated for 14 h at 1200C. Lecithin is a standard for phospholipids. A lipid sample of 10-20 µL fraction was applied at specified spots on the TLC plate and placed in the chamber containing the solvent system. The run was allowed for 10 minutes, after which the plates were air dried, and the resolved lipids were visualized using iodine vapors. Then the Rf value was calculated.
- Hydrogen Peroxide (H2o2) Radical Scavenging Ability
The H2O2 scavenging ability of phospholipid extract was determined according to the method of Ruch et al. A solution of 40 mM H2O2 was prepared in phosphate buffer saline(pH 7.4). To a series of various concentrations of extract (100–200 µg/mL), 1 mL of H2O2 solution (40 mM) was added in the dark. The absorbance of hydrogen peroxide at 230 nm was determined after 10 min using a UV–Visible spectrophotometer against a blank solution. The blank contained the phosphate buffer without H2O2. The results were compared with the percentage scavenging ability of ascorbic acid as a standard (100 µg/mL). All the samples were run in triplicate. The percentage of scavenged H2O2 was calculated using the following equation:
% of scavenged H2O2 = [(Ac − As)/Ac] × 100,
where Ac = Absorbance of control and As = Absorbance in the presence of extract.
- In Vitro Antioxidant Activity: Reducing Power
In vitro, the antioxidant assay was estimated by the ferric reducing antioxidant power (FRAP) method. To 2.5 mL of aliquots of various concentrations of ascorbic acid and 100 to 600 µg/mL of sample, 2.5 mL of 0.2 M phosphate buffer (pH 6.6), and 2.5 mL of 1% potassium ferricyanide (w/v) were added. The reaction mixture was incubated at 50 ◦C for 30 min and was rapidly cooled with running water after the completion of incubation. Later, 2.5 mL of 10% trichloroacetic acid (w/v) was added, and the mixture was centrifuged at 3000 rpm for 10 min. Afterward, 2.5 mL of supernatant was collected and mixed with 2.5 mL of distilled water. The resultant was vortexed for a short time, and 0.5 mL of 0.1% ferric chloride (FeCl3) solution(freshly prepared) was added until the green- colored solution was formed. The mixture was allowed to stand for 10 min, and the absorbance of standard and test samples was measured at 700 nm. Distilled water with no added extracts was used as a blank. Ascorbic acid was used as a standard, and all the samples were run in triplicate.
Results and Discussion
- Isolation of Lipids
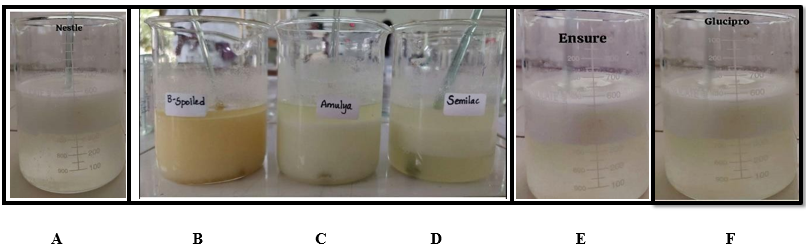
Nestle, B- Dairy whitener powder, C- Amulya, D- Similac, E- Ensure, F- Glucipro diabetic. Milk lipids in the chloroform [bottom layer] are separated from the solidified layer as observed in the above milk powder samples.
Separation of Chloroform Layer

Yield of the Phospholipids
|
S.NO |
Milk Powder |
The yield of lipids (mg/gm of powder) |
|
1 |
Nestle Milk powder, |
8.117 mg |
|
2 |
Dairy whitener |
4.925mg |
|
3 |
Amulya |
7.88mg |
|
4 |
Similac |
6.867mg |
|
5 |
Ensure |
7.238mg |
|
6 |
Glucipro |
5.612mg |
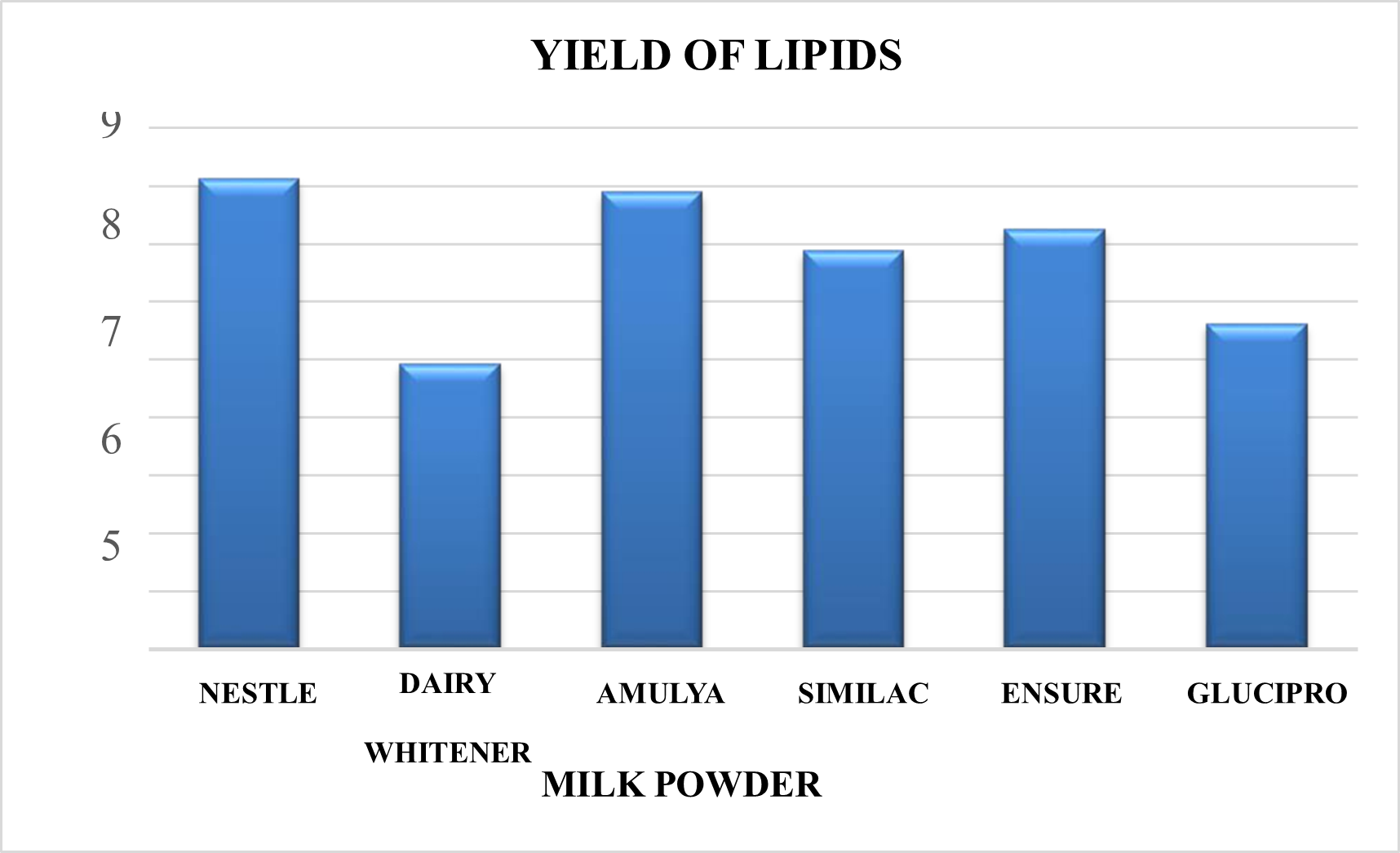
The maximum yield of lipids was identified in the following order: Nestle, Amulya, Ensure, Similac, Glucipro, and Dairy Whitener.
- Identification of Various Phospholipids by Tlc
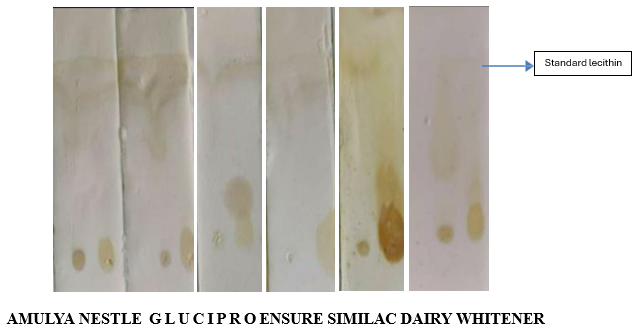
Rf values of the extracted crude lipids separated on TLC
|
Phospholipids/sample |
Standard (Rf) |
Nestle |
Dairy whitener |
Amulya |
Similac |
Ensure |
Glucipro |
|
Lecithin |
0.8 |
0.82 |
- |
0.8 |
0.8 |
0.8 |
0.8 |
|
Sphingomyelin as per Huang etal., 2020 |
0.21 |
0.2 |
0.2 |
- |
- |
- |
0.2 |
|
Phosphatidylethanolamine as per Huang etal., 2020 |
0.88 |
0.81 |
0.8 |
0.8 |
0.8 |
0.8 |
0.82 |
|
Phosphatidylinositol as per Huang etal., 2020 |
0.95 |
0.90 |
- |
0.98 |
0.98 |
0.9 |
0.92 |
All the phospholipids are present in Glucipro and Nestle.
- H2o2 Radical Scavenging Activity
|
Milk Powder |
Crude extract |
After affinity purification |
|
|
100µL |
100 µL |
|
Amulya |
4% |
85% |
|
Nestle |
5% |
76% |
|
Dairy whitener |
11% |
87% |
|
Similac |
6% |
92% |
|
Glucipro |
4% |
71% |
|
Ensure |
5% |
90% |
%- H2O2 Reduced
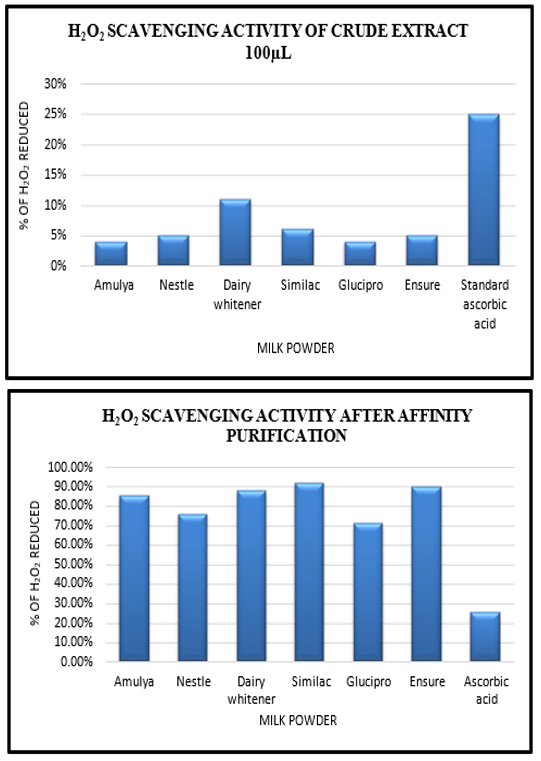
Due to its antioxidant nature, the milk extract has the potential to reduce ROS, such as H2O2. The percentage ability of the milk extract to scavenge H2O2 is shown above in different milk powders. The H2O2 scavenging activity of milk extract increases in a concentration-dependent manner. The maximum percentage of scavenging activity is shown by 100 µg/mL of the milk powder extract. This antioxidant activity proves its correlation with a high content of polyphenolic compounds in the extract.
- In Vitro Antioxidant Activity: Reducing Power
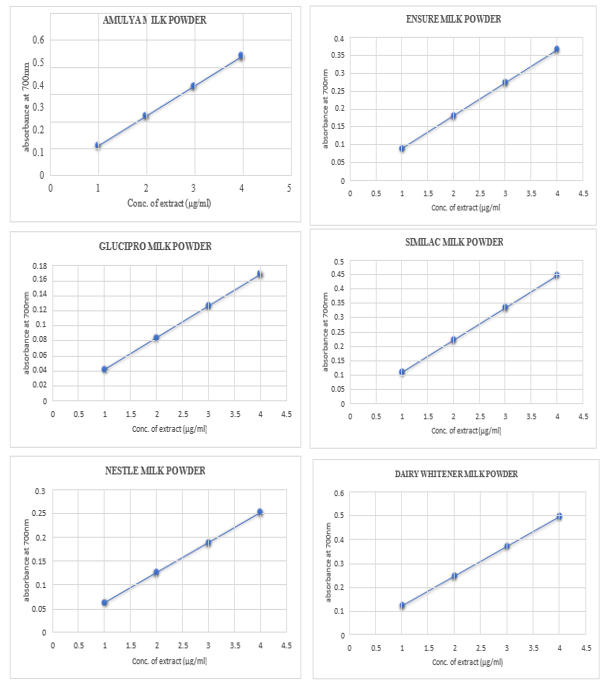
CRUDE EXTRACT (µg/ml)
|
CONC. |
AMULYA |
NESTLE |
GLUCIPRO |
ENSURE |
SIMILAC |
DAIRY WHITENER |
|
10µL |
0.132 |
0.063 |
0.042 |
0.092 |
0.113 |
0.124 |
|
20µL |
0.264 |
0.126 |
0.084 |
0.184 |
0.226 |
0.248 |
|
30µL |
0.396 |
0.189 |
0.126 |
0.276 |
0.339 |
0.372 |
|
40µL |
0.528 |
0.252 |
0.168 |
0.368 |
0.452 |
0.496 |
The reducing power ability of milk powder extract was found to be comparable to the standard. These figures show that the reducing power of milk extract in terms of absorbance was proportionally increasing with an increase in concentration utilized.
AFTER AFFINITY PURIFICATION
|
CONC. |
AMULYA |
NESTLE |
GLUCIPRO |
ENSURE |
SIMILAC |
DAIRY WHITENER |
|
10µl |
0.152 |
0.092 |
0.144 |
0.112 |
0.324 |
0.214 |
|
20µl |
0.304 |
0.184 |
0.288 |
0.224 |
0.648 |
0.428 |
|
30µl |
0.456 |
0.276 |
0.432 |
0.336 |
0.972 |
0.642 |
|
40µl |
0.608 |
0.368 |
0.576 |
0.448 |
1.296 |
0.856 |
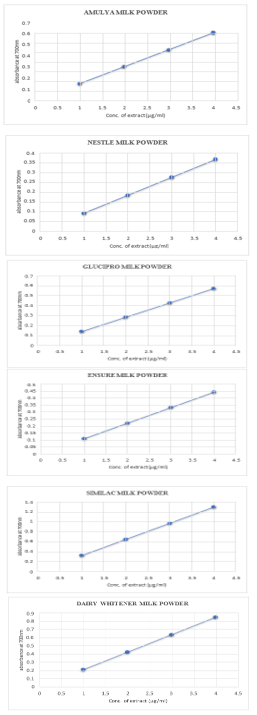
The reducing power ability of milk powder extract was found to be comparable to the standard. These figures show that the reducing power of milk extract in terms of absorbance was proportionally increasing with an increase in concentration utilized.
Discussion
Studies of milk powders represented that Phospholipids identified by TLC indicate the presence of different phospholipids, out of which Glucipro and Nestle had all phospholipids present in them, Huang etal., 2020. The chromatogram of standard lecithin and milk phospholipids and relative position was established by lining up each band's Rf value with that of the reference. The maximum yield of lipids was identified in the following order: Nestle, Amulya, Ensure, Similac, Glucipro, and Dairy Whitener. H2O2 scavenging activity of the crude extract was observed more in dairy whitener, though it has less yield. With the present study, we documented a correlation between the total yield, H2O2 scavenging activity, and ferric reducing power assay and confirmed its antioxidant nature that was not in the following order as seen earlier in TLC, Shehwaz Anwar, etal.,2020.
Conclusion
In conclusion, milk phospholipids present in milk powders play an important key role in both nutritional and technological fields. The TLC method concludes, presence of all phospholipids in glucipro and nestle followed by amulya, and their antioxidant activity was determined by the FRAP method, and Amulya milk powder had the maximum concentration before the column, and Similac milk powder had the maximum concentration after the column. Next, the H2O2 method was used to determine the radical scavenging activity, and the least activity was seen in dairy whitener as the percentage of H2O2 reduced was increased, and the highest activity was seen in glucipro milk powder. These correlations of scavenging activity and ferric reducing antioxidant activity in phospholipids are used for further studies.
- Contarini G, Povolo M. (2013). Phospholipids in milk fat: composition, biological and technological significance, and analytical strategies. Int J Mol Sci. 14(2): 2808-2831.
- Contarini G, Povolo M. (2013). Phospholipids in milk fat: composition, biological and technological significance, and analytical strategies. Int J Mol Sci. 14(2): 2808-2831.
- Küllenberg, D, Taylor, L. A, Schneider, M, Massing, U. (2012). Health effects of dietary phospholipids. Lipids in health and disease. 11: 1-16.
- Pimentel, L, Gomes, A, Pintado, M, Rodríguez-Alcalá, L. M. (2016). Isolation and analysis of phospholipids in dairy foods. Journal of Analytical Methods in Chemistry. 2016:9827369.
- Khan, I. T, Nadeem, M, Imran, M, Ullah, R, Ajmal, M, Jaspal, M. H. (2019). Antioxidant properties of Milk and dairy products: a comprehensive review of the current knowledge. Lipids in health and disease. 18(1): 41.
- Küllenberg, D, Taylor, L.A, Schneider, M. (2012). Health effects of dietary phospholipids. Lipids Health Dis. 11: 3.
- Khan, I.T, Nadeem, M, Imran, M. (2019). Antioxidant properties of Milk and dairy products: a comprehensive review of the current knowledge. Lipids Health Dis. 18: 41.
- Khan IT, Nadeem M, Imran M, Ullah R, Ajmal M, Jaspal MH. (2019). Antioxidant properties of Milk and dairy products: a comprehensive review of the current knowledge. Lipids Health Dis. 18(1): 41.
- Küllenberg de Gaudry, Daniela & Taylor, Lenka & Schneider, Michael & Massing, Ulrich. (2012). Health effects of dietary phospholipids. Lipids in health and disease. 11. 3. 10.1186/1476-511X-11-3.
- Medically reviewed by Katherine Marengo LDN, R.D. (2020). Nutrition — By Jillian Kubala, MS, RD.
- riordanclinic.org
- Schverer, M, O'Mahony, S. M, O'Riordan, K. J, Donoso, F, Roy, B. L, Stanton, C, Dinan, T. G, Schellekens, H, Cryan, J. F. (2020). Dietary phospholipids: Role in cognitive processes across the lifespan. Neuroscience and biobehavioral reviews. 111: 183–193.
Download Provisional PDF Here
PDF




p (1).png)




.png)




.png)
