Review Article
Neurogenesis in the Hippocampus and Its Influence on Cognitive and Emotional Abilities
- Elizaveta I Bon
Corresponding author: Elizaveta I Bon, Candidate of biological science, Assistant professor of pathophysiology department named D. A. Maslakov, Grodno State Medical University; Grodno State Medical University, 80 Gorky St,230009, Grodno, Belarus.
Volume: 2
Issue: 3
Article Information
Article Type : Review Article
Citation : Bon E.I, Maksimovich N.Ye, Zimatkin S.M, Yusko E.V. Neurogenesis in the Hippocampus and Its Influence on Cognitive and Emotional Abilities. Journal of Medical and Clinical Case Reports 2(3). https://doi.org/10.61615/JMCCR/2025/JULY027140719
Copyright: © 2025 Elizaveta I Bon. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
DOI: https://doi.org/10.61615/JMCCR/2025/JULY027140719
Publication History
Received Date
03 Jul ,2025
Accepted Date
15 Jul ,2025
Published Date
19 Jul ,2025
Abstract
The neurogenesis of the hippocampus in adults plays a key role in cognitive, emotional, and behavioral functions, sparking discussions about its evolutionary significance. Differences between neurogenesis during development and in adulthood are emphasized, suggesting that they serve different functions: the constitution and lifelong adaptation of cognitive and emotional behavior. This distinction also indicates varying vulnerabilities to mental disorders depending on the life stage. We propose new research directions focused on the impact of hippocampal neurogenesis on mental disorders, which could deepen the understanding of risk factors and adaptation mechanisms.
Keywords: hippocampus, neurons, neurogenesis.
►Neurogenesis in the Hippocampus and Its Influence on Cognitive and Emotional Abilities
Bon E.I1*, Maksimovich N.Ye2, Zimatkin S.M3, Yusko E.V4
1,2,3,4 Grodno State Medical University, Gorkogo St, Grodno, Republic of Belarus.
Relevance
In most mammals, new neurons are formed not only during the embryonic period but also after birth. Soon after the discovery of neurogenesis in adults, it was noted that the addition of new neurons affects cognitive functions, particularly memory. Similarly, late neurogenesis also impacts affective functions, although this fact has been acknowledged with difficulty. Current data show that new neurons can arise not only from stem cells but also from neuroblasts, which exhibit prolonged maturation and may participate in functional brain circuits under certain signals. The importance of adding new neurons to brain networks in adults is discussed, especially in the context of affective outcomes.[1] It is also emphasized that adult neurogenesis may be a finite cellular process that incorporates elements from both the internal and external environment to regulate brain functions. Research on neurogenesis in humans offers new insights into people as mammals. This ability could serve as a foundation for treating mental disorders, cognitive impairments, and age-related diseases. The vision of an adult brain that continually integrates new neurons represents an important step toward developing new therapeutic interventions for treating mental disorders associated with high morbidity, mortality, and social costs.[2]
Objective
To investigate the influence of new neurons in the hippocampal formation on the affective functions of the brain in adult mammals, including humans, and to assess the potential of neurogenesis as a means to improve mental health and treat cognitive disorders.
Methodology
The foundation of this study was a review of contemporary foreign and domestic literature on this topic.
Results of the Study and Discussion
The hippocampus consists of densely packed cells arranged in a ribbon-like structure that extends along the medial walls of the lower horns of the lateral ventricles of the brain in an anteroposterior direction. Both halves of the hippocampus are connected by commissural nerve fibers. The hippocampal formation is divided into the "proper hippocampus" (fields CA1, CA2, and CA3), the dentate gyrus, and the subiculum.[2] The proper hippocampus is further divided into proximal large-cell and distal small-cell areas, with fields CA3 and CA2 corresponding to the large-cell area, and CA1 to the small-cell area. The ventricular surface of the hippocampus is called the alveus. The alveus is a thin layer of white matter formed by the axons of the pyramidal cells of the hippocampus, covered by a layer of ependymal cells. The fibers of the alveus converge at the medial edge of the hippocampus, forming the fimbria of the hippocampus, which in turn gives rise to the fornix.[3]
According to modern histological nomenclature, the proper hippocampus is distinguished into three layers: 1) molecular (stratum moleculare), which includes the eumolecular (substratum eumoleculare), lacunar (substratum lacunosum), and radial (substratum radiatum) sublayers; 2) pyramidal (stratum pyramidale); and 3) oriens (stratum oriens) layers. (Figure 1). The structure of these layers is generally consistent across all fields of the hippocampus. (Table 1) [5,6,7]
Table 1. – Neuronal and Transmitter Organization of the Hippocampus
|
Name of the neuron |
Layers (sub-layers) of the cortex. |
Afferent innervation. |
Efferent innervation.
|
Mediator |
|
Non-pyramidal interneurons. |
Molecular (stratum molecular). |
Axons of neurons from the entorhinal cortex and thalamic nuclei, non-pyramidal interneurons in the molecular and marginal layers. |
Non-pyramidal interneurons in the molecular and marginal layers, dendrites of pyramidal neurons. |
GABA |
|
Non-pyramidal interneurons. |
Lacunar sublayer (substratum lacunosum). |
Non-pyramidal interneurons in the molecular and marginal layers. |
–//– |
GABA |
|
Non-pyramidal interneurons. |
Radial sublayer (substratum radiatum). |
Non-pyramidal interneurons of the molecular and marginal layers. |
–//– |
GABA |
|
Pyramidal neurons. |
Pyramidal (stratum pyramidale). |
Axons of granule neurons of the dentate gyrus, Schaffer collaterals, axons of basket cells, and all other neurons of the molecular and marginal layers. |
Schaffer collaterals, neurons of the entorhinal cortex, and basket neurons. |
Acetylcholine. |
|
Basket neurons. |
Pyramidal. |
Axons of pyramidal neurons. |
Pyramidal neurons. |
GABA |
|
Trilaminar neurons. |
Pyramidal. |
–//– |
Neurons of the subiculum |
GABA |
|
Candelabrum cells. |
Pyramidal. |
–//– |
Pyramidal neurons. |
GABA |
|
Non-pyramidal interneurons. |
Stratum oriens. |
Non-pyramidal interneurons of the molecular and stratum oriens layers. |
Pyramidal neurons. |
GABA |
In the molecular layer, there are three types of non-pyramidal GABAergic neurons [7]. In the eumolecular sublayer, there lies a bundle of fibers directed from the subiculum, ending afferent pathways from the entorhinal cortex and the nuclei of the mid-thalamus, while in the lacunar sublayer, axons from the hippocampus to the subiculum pass through. In the CA3 field, unlike in the CA2 and CA1 fields, there is a narrow acellular zone located just above the layer of pyramidal neurons, where the axons of the granule cells (substratum lucidum) run. At the distal end, these fibers form a bend that marks the boundary between the CA3 and CA2 fields. The substratum radiatum includes nerve fibers that establish connections between the neurons of the CA3 and CA1 fields. The pyramidal layer is the main layer of the hippocampus itself. It contains pyramidal, basket, tri-laminar neurons, and chandelier cells. The dendrites of pyramidal cells are directed towards both the molecular and the marginal layers [8]. Lorente de Nó (1934) noted differences in the dendritic organization of pyramidal cells in different parts of CA3 and CA1 and used this to further divide these fields into three subregions (CA3a, b, c; CA1a, b, c) [10].
Figure 1. Diagram of the neuronal organization of the rat hippocampus (according to data from the US National Library of Medicine) [14].
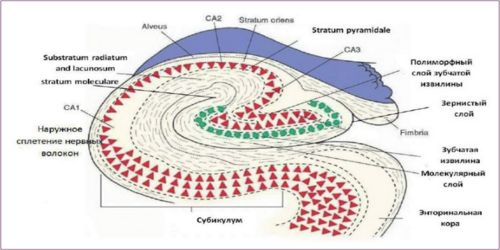
The narrow, relatively acellular marginal layer contains the basal dendritic branches of pyramidal neurons, as well as the bodies and branches of the dendrites of polymorphic (non-pyramidal) interneurons. In the hippocampus itself, eight types of neurons are distinguished. The main ones, pyramidal neurons, are cholinergic, while the others are GABAergic. (Table 1) Pyramidal neurons are located in the pyramidal layer. [11,12] Their sizes and organization in CA3 change sequentially depending on their position along the CA3 axis. Cells in the proximal part, located near the dentate gyrus, have the smallest dendritic branches, while neurons in the distal part of the CA3 field (near CA2) have the largest dendritic branches. CA2 contains a mixed population of both neurons with extensive dendritic branching, similar in size to those in the distal part of CA3, as well as cells with smaller dendritic branches resembling pyramidal neurons of CA1. 42-51% of the basal dendrites of CA3 pyramidal neurons are located in the stratum oriens, with 34% of the dendritic branches of CA1 neurons also extending into this layer, while 18% of the apical dendrites from the same fields go into the stratum moleculare and lacunosum. In addition to pyramidal neurons, there is a heterogeneous population of basket cells of various sizes and shapes in the pyramidal layer of the hippocampus. They have both apical and basal dendritic branches. [13,14] The axons of basket neurons extend transversely from the cell bodies and form basket-like networks that synapse with the bodies of hippocampal pyramidal neurons. Basket neurons receive excitatory impulses from pyramidal neurons and exert inhibitory effects on them. Pyramidal cells generate recurrent excitation, which is an important mechanism for memory formation. (Table 1)
There are different types of non-pyramidal interneurons. They are located in the molecular and marginal layers. The vast majority of them are considered local circuit neurons. The dendrites of hippocampal interneurons extend into the stratum oriens, while their axons form synapses in the stratum moleculare. These cells synapse with the dendrites of pyramidal neurons, exerting inhibitory effects on them. (Table 1) Some of the interneurons in the CA1 field have quite extensive axonal branching along the transverse axis of the hippocampus, reaching the CA3 field and the dentate gyrus. These cells are typically found in the marginal layer, with their dendrites branching in a horizontal plane.[15] The axons of these neurons form symmetric synapses on the dendrites of pyramidal neurons, providing inhibitory feedback. Despite the wealth of information about the structure of hippocampal interneurons that has emerged over the past decade, their functions are still not fully understood. Interneurons can excite or inhibit other cells and have an extremely variable number of excitatory and inhibitory inputs. Furthermore, the overall effect of interneuronal transmission can vary depending on the type of interneuron and the postsynaptic structure with which it forms a synaptic contact. Most of the non-pyramidal cells are GABAergic and exert an inhibitory influence on cholinergic pyramidal neurons in the hippocampus. (Table 1) In addition, there are interneurons in the hippocampus with additional terminal branching in the pyramidal layer—trilaminar neurons. [16,17] Their dendrites encircle the dendrites of pyramidal neurons, while their axons form extrahippocampal synapses. The perikarya of other interneurons are located in the pyramidal or radial layer and have a rather limited local network of axons that form synapses with the dendrites of pyramidal cells. All fields of the hippocampus in the pyramidal layer also contain chandelier cells. Their dendrites form synapses on the dendrites of pyramidal neurons, while their axons innervate the initial axonal segment of pyramidal neurons.
Evolutionary considerations are necessary but insufficient for characterizing the function of hippocampal neurogenesis in adults (AHN). The hippocampus is involved in various functions such as learning and memory, emotion regulation, attention processes, and motivational states, to name just a few. It is a key structure for processing information about events and contextual elements, known as episodic memory, and it is highly sensitive to stress, drugs, and aging.[18]
It is important to note that the development of memory and emotional responses is not a singular ontogenetic process but follows a precise timeline. Thus, the ability to learn emerges sequentially from simple to complex. For example, non-associative learning occurs before associative learning; the emergence of conditioned fear responses precedes the development of the conditioned blinking reflex; egocentric learning precedes allocentric learning in spatial navigation tasks, where proximal signals are used before distal ones; and backward learning occurs last. Interestingly, in infancy, memories appear to be easily and quickly forgotten, a process known as "childhood amnesia." Recent studies have shown that childhood memories are not actually lost and that the transition to adult-like memory (around postnatal day 25, PND25) is associated with the maturation of the hippocampus. [11,12]
In adulthood, new neurons are continually added in the dentate gyrus (DG), and their function is thought to involve the modulation and adaptation of fundamental behaviors acquired during development. Regarding the functioning of the hippocampus, this is supported by the existence of binding capabilities that allow adult animals not only to recognize a specific object or location but also to associate a particular element with emotional valence, as well as specific place, context, and time (what, where, when—memory, i.e., experience-based memory). This includes the ability to encode various sensory (visual, auditory, olfactory) and interoceptive information, to link them into a unique representation, and to adequately integrate them (by comparing with previously acquired information) to support deductive reasoning. This is the process by which existing memories are retrieved and recombined to handle new situations. It requires the flexible use of learned information and relies on both the ability to form distinct representations in memory that share overlapping elements during encoding and the ability to extract them from partial input data.[17]
Thus, the functions of developmental hippocampal neurogenesis (DHN) and adult hippocampal neurogenesis (AHN) are both distinct and interconnected.
AHN plays an important role in emotional states, both positive and negative. When emotions are positive, they motivate goal achievement or reward. G supports the reward process, as evidenced by the existence of selective reward cells that coordinate CA3 neuronal activity to regulate behavior. Similar to natural stimuli (food, physical activity, and sexual activity), drugs are highly rewarding and enhance stimulus-response associations. When emotions are negative, they have evolved as a defense mechanism, allowing adaptation to potential threats, as seen in cases of anxiety. It consists of a complex cognitive, affective, physiological, and behavioral response system related to preparation for expected events or circumstances perceived as threatening. Both DHN and AHN are key elements in constructing a behavioral response adapted to environmental conditions—DHN through the early establishment of a repertoire of basic elements or modules necessary for this adapted response, and AHN through lifelong binding of these modules in appropriate combinations. When disrupted, these two functions may be involved in mental disorders. [18,19,20]
Conclusion
Natural selection has equipped many animals with the capacity to face the unpredictable variety of situations they will encounter throughout their lives. Adaptation abilities are hereditary; some rules are passed on through learning; however, behavioral adjustment is not and cannot be genetic. There is substantial evidence that these adaptive abilities are generally supported by neurogenesis in the DG. Most authors discussing the specific function of AHN have not reached a consensus. This, in turn, strongly suggests that a clear distinction should be made between the respective roles of DHN and AHN in the emergence of adapted or maladapted behavior. It is expected that developmental resilience will be associated with a repertoire of basic reactions—such as how intensely and easily an animal can experience fear—and will remain largely unchanged throughout life.[20,21,22] Adult resilience is expected to be linked to how adults associate memories, emotions, and behaviors based on past and present experiences—such as determining when to or not to feel fear—and will be subject to some degree of change over their lifetime. There is already substantial evidence that AHN and DHN, and their disruptions, play different roles in the risk of adapted or maladapted behavior. It may still be too early to discuss the implications of the theoretical framework proposed in this review for clinical and therapeutic development. [23,24] It is sufficient to say that it potentially questions the significance of certain differences between mental disorders and offers hypotheses about their underlying mechanisms and what can be done to alleviate them. Events such as disasters and pandemics demonstrate how human adaptive abilities save lives while simultaneously generating stress. Thus, just as some of us can maximize the benefits from this and wonderfully adapt to many circumstances, for others it becomes a burden of developmental vulnerability in adulthood. [25,26]
- Bon E. I, Maksimovich N. E, Valko N. A. (2022). The Brain of the Rat (Review) // Orenburg Medical Bulletin. 10.2(38): 5-11.
- Zimatkin S. M, Bon E. I. (2013). Cellular and Molecular Mechanisms of Alcohol's Damaging Effects on Brain Development // Proceedings of the National Academy of Sciences of Belarus. Series of Medical Sciences. 2: 109-117.
- Bon E. I, Maksimovich N. E, Zimatkin S. M. (2020). Morphological Disorders of Hippocampal Neurons in Rats with Subtotal and Total Ischemia // Orenburg Medical Bulletin. 8.2(30): 41-46.
- Bon E. I, Maksimovich N. E. (2021). Comparative Analysis of Morphological Disorders in Neurons of the Parietal Cortex and Hippocampus of Rats under Different Types of Experimental Cerebral Ischemia // Orenburg Medical Bulletin. 9.2(34): 29-37.
- Bon’ E. I, Zimatkin S. M. (2018). Structure and Development of the Hippocampus in Rats // Grodno State Medical University Journal. 16(2): 132-138.
- Bon E. I, Maksimovich N. E, Zimatkin S. M. (2018). Cytochemical Disorders in the Parietal Cortex and Hippocampus of Rats After Subtotal Ischemia // Bulletin of Vitebsk State Medical University. 17(1): 43-49.
- Bon’E. I, Zimatkin S. M. (2018). Structural and Neurotransmitter Organization of Different Areas of the Cerebral Cortex // Bulletin of Smolensk State Medical Academy. 17(2): 85-92.
- Bon E. I. (2024). CONTENT OF HEAT SHOCK PROTEIN HSP70 IN NEURONS OF THE PARIETAL CORTEX AND HIPPOCAMPUS OF RATS WITH CEREBRAL ISCHEMIA OF VARYING SEVERITY // Ulyanovsk Medical and Biological Journal. 3: 117-122.
- Abbott L. F. (2020). The mind of a mouse //Cell. 182(6): 1372-1376.
- Abrous, Djoher Nora. (2022). “A Baldwin interpretation of adult hippocampal neurogenesis: from functional relevance to physiopathology.” Molecular psychiatry. 27(1): 383-402.
- Aimone, James B. (2010). “Adult neurogenesis: integrating theories and separating functions.” Trends in cognitive sciences. 14(7): 325-337.
- Anacker, Christoph, and René Hen. (2017). “Adult hippocampal neurogenesis and cognitive flexibility - linking memory and mood.” Nature reviews. Neuroscience. 18(6): 335-346.
- Anacker C. (2018). Hippocampal neurogenesis confers stress resilience by inhibiting the ventral dentate gyrus //Nature. Т. 559(7712): 98-102.
- Ben Abdallah, Nada M-B. (2010). “Early age-related changes in adult hippocampal neurogenesis in C57 mice.” Neurobiology of aging. 31(1): 151-161.
- Bienkowski, Michael S. (2018). “Integration of gene expression and brain-wide connectivity reveals the multiscale organization of mouse hippocampal networks.” Nature neuroscience. 21(11): 1628-1643.
- Brenowitz, Eliot A, and Tracy A Larson. (2015). “Neurogenesis in the adult avian song-control system.” Cold Spring Harbor perspectives in biology. 7(6): 019000.
- Clelland, C D. (2009). “A functional role for adult hippocampal neurogenesis in spatial pattern separation.” Science (New York, N.Y.). 325(5937): 210-213.
- Denny, Christine A. (2012). “4- to 6-week-old adult-born hippocampal neurons influence novelty-evoked exploration and contextual fear conditioning.” Hippocampus. 22(5): 1188-1201.
- Freund, Julia. (2013). “Emergence of individuality in genetically identical mice.” Science (New York, N.Y.). 340(6133): 756-759.
- Garthe, Alexander. (2016). “Mice in an enriched environment learn more flexibly because of adult hippocampal neurogenesis.” Hippocampus. 26(2): 261-271.
- Gu, Yan. (2012). “Optical controlling reveals time-dependent roles for adult-born dentate granule cells.” Nature neuroscience. 15(12): 1700-1706.
- Hvoslef-Eide M, Oomen C. A. (2016). Adult neurogenesis and pattern separation in rodents: a critical evaluation of data, tasks and interpretation //Frontiers in Biology. Т. 11(3): 168-181.
- Kempermann, Gerd. (2010). “Why and how physical activity promotes experience-induced brain plasticity.” Frontiers in neuroscience. 4: 189.
- Knoth, Rolf. (2010). “Murine features of neurogenesis in the human hippocampus across the lifespan from 0 to 100 years.” PloS one. 5(1): 8809.
- Lemaire, V. (1999). “Behavioural trait of reactivity to novelty is related to hippocampal neurogenesis.” The European journal of neuroscience. 11(11): 4006-4014.
- Lods, Marie. (2021). “Adult-born neurons immature during learning are necessary for remote memory reconsolidation in rats.” Nature communications. 12(1): 1778.
Download Provisional PDF Here
PDF




p (1).png)




.png)




.png)
