Research Article
RESULTS OF XLIF SURGERY FOR SPINAL STENOSIS AND LUMBAR SPONDYLOLISTHESIS
- Dr. Tan Minh Hoang
Corresponding author: Tan Minh Hoang, Hanoi Medical University, Hanoi, Vietnam
Volume: 2
Issue: 1
Article Information
Article Type : Research Article
Citation : Tan Minh Hoang, Hung Dinh Kieu, Vu Nguyen, Trung Kien Tran, Tan Chor Ngee, Ha Dai Duong. Results of Xlif Surgery for Spinal Stenosis and Lumbar Spondylolisthesis. Journal of Medical and Clinical Case Reports 2(1). https://doi.org/10.61615/JMCCR/2025/FEB027140210
Copyright: © 2025 Tan Minh Hoang. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
DOI: https://doi.org/10.61615/JMCCR/2025/FEB027140210
Publication History
Received Date
20 Jan ,2025
Accepted Date
04 Feb ,2025
Published Date
10 Feb ,2025
Abstract
Background
To evaluate clinical and imaging results after surgery for spinal stenosis and spondylolisthesis using a lateral approach.
Methods
33 patients with 36 segments of surgery diagnosed with lumbar spinal stenosis and spondylolisthesis were surgically treated with the XLIF method. Clinical outcomes measured included VAS scores for lower back pain and leg pain, ODI, and JOA scores. Magnetic resonance imaging of the lumbar spine after surgery was used to evaluate indirect decompression. X-ray to evaluate segmental lordosis and lumbar lordosis after surgery. X-ray or CT scan to evaluate bone fusion after 6 months of surgery.
Results
There were 33 patients with 36 segments of surgery. There were 14 males and 19 females with an average age of 57.58±8.573 (36-74). There was significant improvement in VAS for lower back pain from 7.19±1.75 to 2.63±1.66, VAS for leg pain from 6.75±2.09 to 1.19±1.77, ODI from 26.78±9.29 to 14.09±8.56, and JOA score from 7.38±2.95 to 13.75±2.06. A-P diameter increased 128%, lateral diameter increased 117%, lateral recess depth increased 156%, disc height increased 128%, foraminal height increased 121%, and spinal canal area increased 124%. The p-values were all <0.001. The average hospital stay was 6.09±2.765 days. Complications included 1 pedicle screw malformation, 1 ALL avulsion fracture, 1 abdominal herniation, 1 venous damage, and 1 failure.
Conclusion
XLIF surgery is an effective option for patients with lumbar spinal stenosis and spondylolisthesis. This is a minimally invasive surgical method that reduces pain, reduces bleeding, and is effective in indirectly decompressing the spinal canal both clinal and imaging.
Keywords: Clinical, imaging, lumbar stenosis, spondylolisthesis, lateral approach surgery.
Abbreviation
XLIF: eXtreme lateral interbody fusion
VAS: Visual analog score
ODI: Oswestry Disability Index
JOA: Japanese Orthopedic Association
MRI: Magnetic resonance imaging
►RESULTS OF XLIF SURGERY FOR SPINAL STENOSIS AND LUMBAR SPONDYLOLISTHESIS
Tan Minh Hoang1*, Hung Dinh Kieu1, Vu Nguyen1, Trung Kien Tran1, Tan Chor Ngee2, Ha Dai Duong1
1Hanoi Medical University, Hanoi, Vietnam.
2Putrajaya hospital, Putrajaya, Malaysia.
Introduction
Extreme lateral interbody fusion (XLIF) surgery is defined as minimally invasive lateral, retroperitoneal surgery to the anterior spinal column with reduced injury to muscles and adjacent structures by manual dissection of the retroperitoneal space. Firstly, McAfee described a minimally invasive retroperitoneal approach for lumbar spinal fusion in 1998[1], which involved psoas muscle retraction posteriorly to expose the disc space. This approach did not require mobilization of abdominal contents and great vessels, avoided extensive muscle stripping and denervation, and left normal spinal stabilizing elements intact (e.g. anterior and posterior longitudinal ligaments), thus avoiding many of the complications associated with anterior and posterior approaches to spinal fusion.
We conducted initial guidance of the psoas muscle to the surface of the psoas muscle, use of the intraoperative neurophysiology monitoring when passing through the psoas muscle, extension of the retraction system, and direct observation of the surgical field, placement of a large interbody instrument to open maximum intervertebral and orthopedic space expansion. XLIF is indirect decompression surgery and thus restoring disc and foraminal height resulting in symptomatic relief is its main advantage over more invasive decompression and interbody fusion surgeries. Indeed, XLIF surgery can reduce post-operative pain, entry wounds, tissue trauma, operating, recovery, and mobility times resulting in shorter hospital stays.
We conducted research on the topic " Results of XLIF surgery for spinal stenosis and lumbar spondylolisthesis" with the aim of:
Evaluating the clinical and imaging effectiveness of lateral interbody bone graft surgery and posterior percutaneous screws to treat lumbar stenosis and spondylolisthesis.
Methods
Patient Selection
Our study recruited 33 patients with 36 segments of surgery who were treated with the XLIF method from 2019 to August 2024. Ethical approval was received from the institution’s review board (IRB approval number 853/GCN-HDDDNCYSH-ĐHYHN)
The indications for XLIF include patients with lumbar spinal stenosis, spondylolisthesis grade 1-2 except for patients with paralysis or severe leg pain at rest, the absence of a free disc fragment on MRI, bony lateral recess, deformities of both lower extremities, diseases that greatly affect diagnosis (spinal tuberculosis, spinal arachnoiditis, etc.), or patients were previously performed lumbar spinal surgery or patients with no clinical manifestations or enable to follow-up post-surgery.
Research Methods
We conducted a cross-sectional descriptive study during the mentioned period time. The demographic and clinical data were retrieved from medical chart reviews. Clinical presentations and imaging investigations were collected before, during, and after surgery.
During the operation, we collected several indexes including intraoperative monitoring of surgery time, amount of blood loss, amount of blood transfusion, and accuracy of screws and cages. We used an MVM5 nerve monitoring system intra-operation. Treatment outcomes were evaluated at post-operation, 1 month, and 6 months after the surgery. The outcome measures included the VAS for lower leg pain and back pain, the ODI for disability, and the JOA scores for functional recovery. All patients had plain anteroposterior (AP) and lateral x-rays, dynamic flexion-extension lateral x-rays before the surgery, at 1 month and 6 months after surgery. All patients had a lumbar spine magnetic resonance imaging study before the surgery, and some of them had lumbar spine MRI after surgery. We measure the anterior and posterior diameter of the spinal canal, lateral diameter, lateral recess depth, spinal canal area, disc height, and foraminal height pre-operation and post-operation on PACS software [2]. X-ray before and after surgery to evaluate segmental lordosis and lumbar lordosis (Figure 4). CT or X-ray of the lumbar spine was arranged 6 months after the surgery to evaluate the bone fusion status. Reconstruction images on the sagittal and coronal planes were used to evaluate the formation of bridging bone. Fusion results were classified from grade I to grade IV using the Bridwell interbody fusion grading system [3]. Grade I or grade II fusion was defined as successful fusion. Evaluation of the success or failure of decompression surgery based on postoperative clinical symptoms. Indirect decompression surgery is considered to have failed when direct decompression surgery is required afterward.
Independent T-tests were used to compare the continuous variables between groups. The chi-square test was used to compare categorical variables between groups. A p-value of < 0.05 was considered statistically significant. The data was processed using SPSS 20.0 software.
Results
This study included 14 males and 19 females with an average age of 57.58±8.573 years (range 36–74 years). These patients received 36 segments of XLIF, including 1-segment fusion in 30 patients, and 2-segment fusion in 3 patients. L4–5 was the most frequently involved level, followed by L3–4, and L2–3. The average hospital stay was 6.09 days (range, 3–14 days). No patient required a blood transfusion. After surgery, the VAS for lower back pain had improved from 7.19±1.75 to 2.63±1.66, and the VAS for leg pain improved from 6.75±2.09 to 1.19±1.77. After one month the ODI had improved from 26.78±9.29 to 14.09±8.56. The JOA score had improved from 7.38±2.95 to 13.75±2.06. All these improvements were statistically significant from baseline with p < 0.001. Complications included 1 pedicle screw malformation (3%), 1 ALL avulsion fracture (3%), and 1 abdominal herniation (3%), 1 failure, 1 venous damage (3%). Reoperation was required in 2 patients for posterior decompression and adjusting the pedicle screw. There was no pedicle screw loosening or posterior cage migration. The demographic data and clinical outcomes were summarized in Tables 1 and 2.
Thirteen patients with 14 fusion segments underwent CT or XQ scan evaluation six months after surgery. Based on the Bridwell grading system, the fusion results were grade I in 6 segments (42.9%), and grade II in 8 segments (57.1%). Successful fusion was achieved in 14 segments (100%).
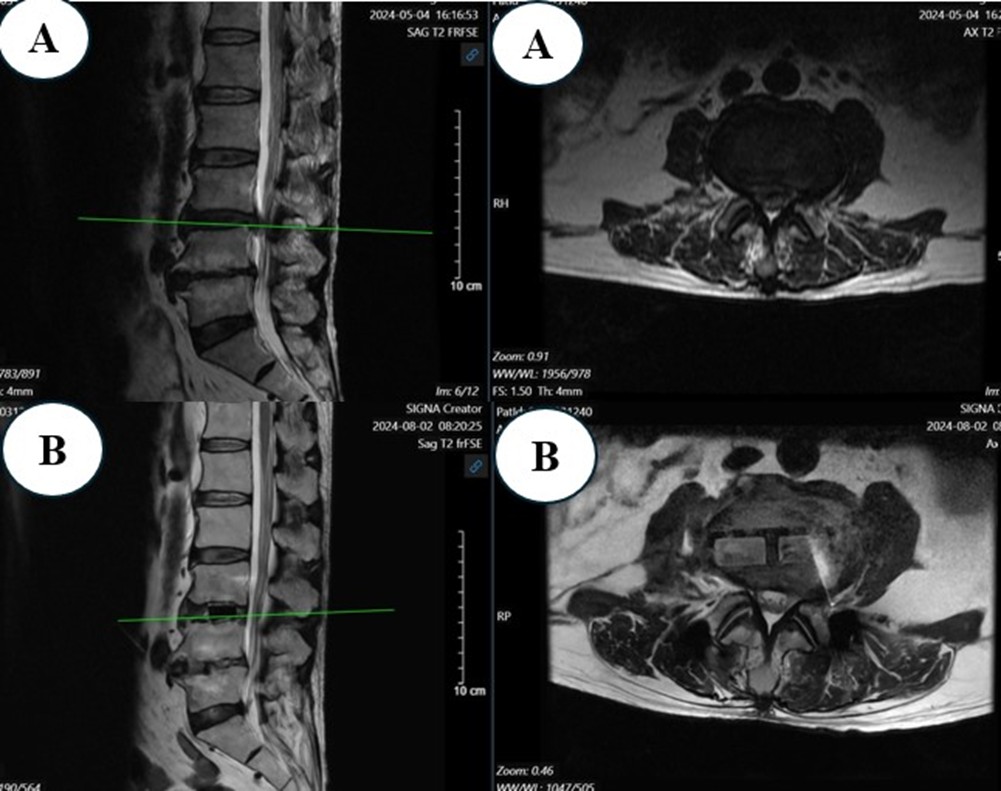
27 segments of surgery underwent MRI after surgery to evaluate the size of the spinal canal. In those 27 segments of surgery, the anterior and posterior diameter increased from 7.48±1.98 mm to 9.61±2.83mm, 128% of preoperation, the lateral diameter increased from 13.06±2.99mm to 15.36±3.29mm, 117% of preoperation lateral recess depth increased from 2.01±1.36 mm to 3.15±0.99 mm, 156% of preoperation, spinal canal area increased from 85.00±34.02 mm2 to 105.39±41.73 mm2, 124% of preoperation, disc height increased from 8.59±2.32 mm to 11.04±2.02 mm, 128% of preoperation, foraminal height increased from 16.42±3.68 mm to 19.88±2.69 mm, 121% of preoperation (Figure 1 and 2). All differences are statistically significant with p<0.001.
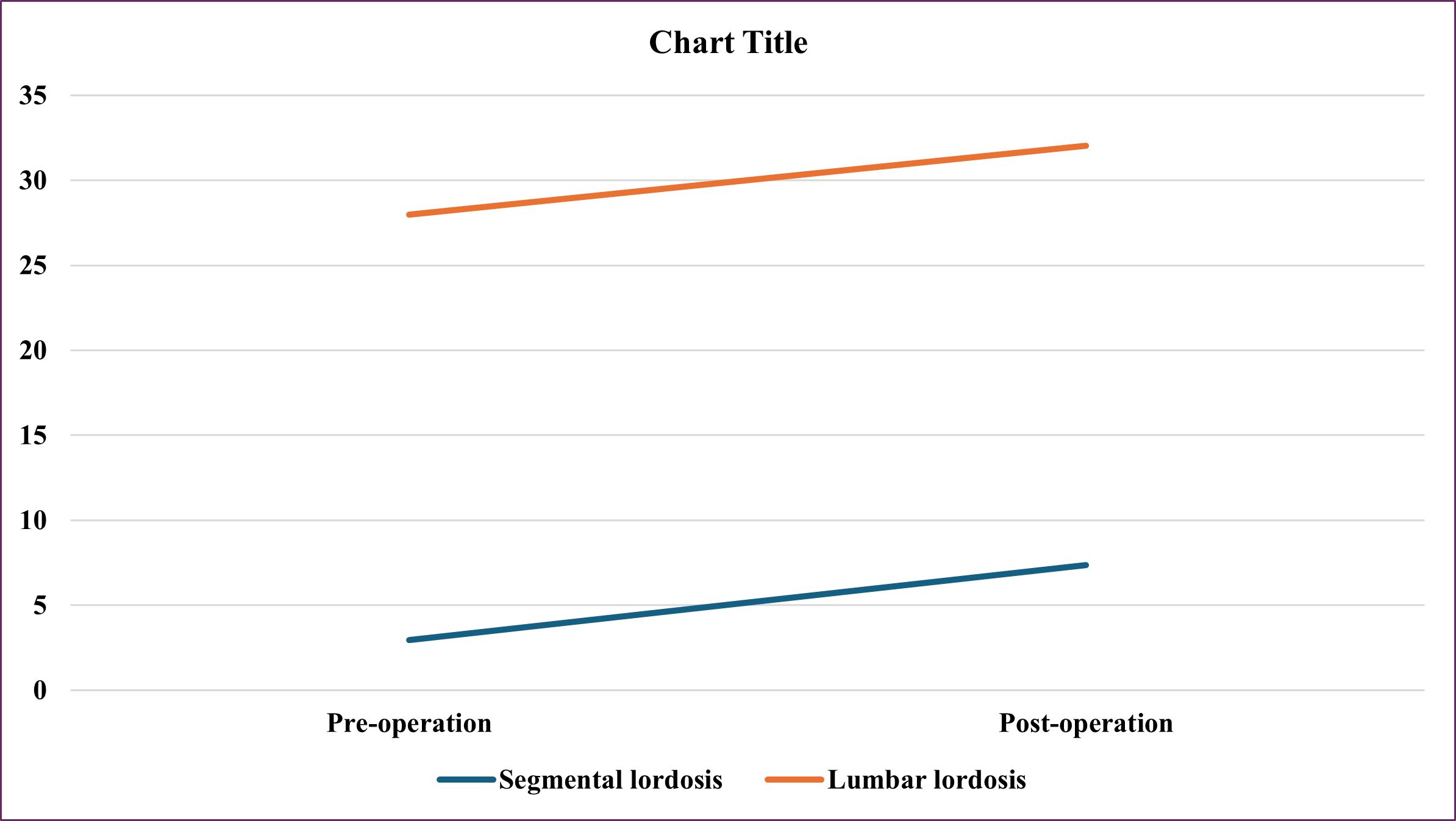
Segmental lordosis increased from 2.95±4.54 to 7.35±3.42, lumbar lordosis increased from 27.97±14.61 to 32.05±12.52 (Figure 3)
Figure 1: Change MRI In the Spinal Canal Before and After Surgery
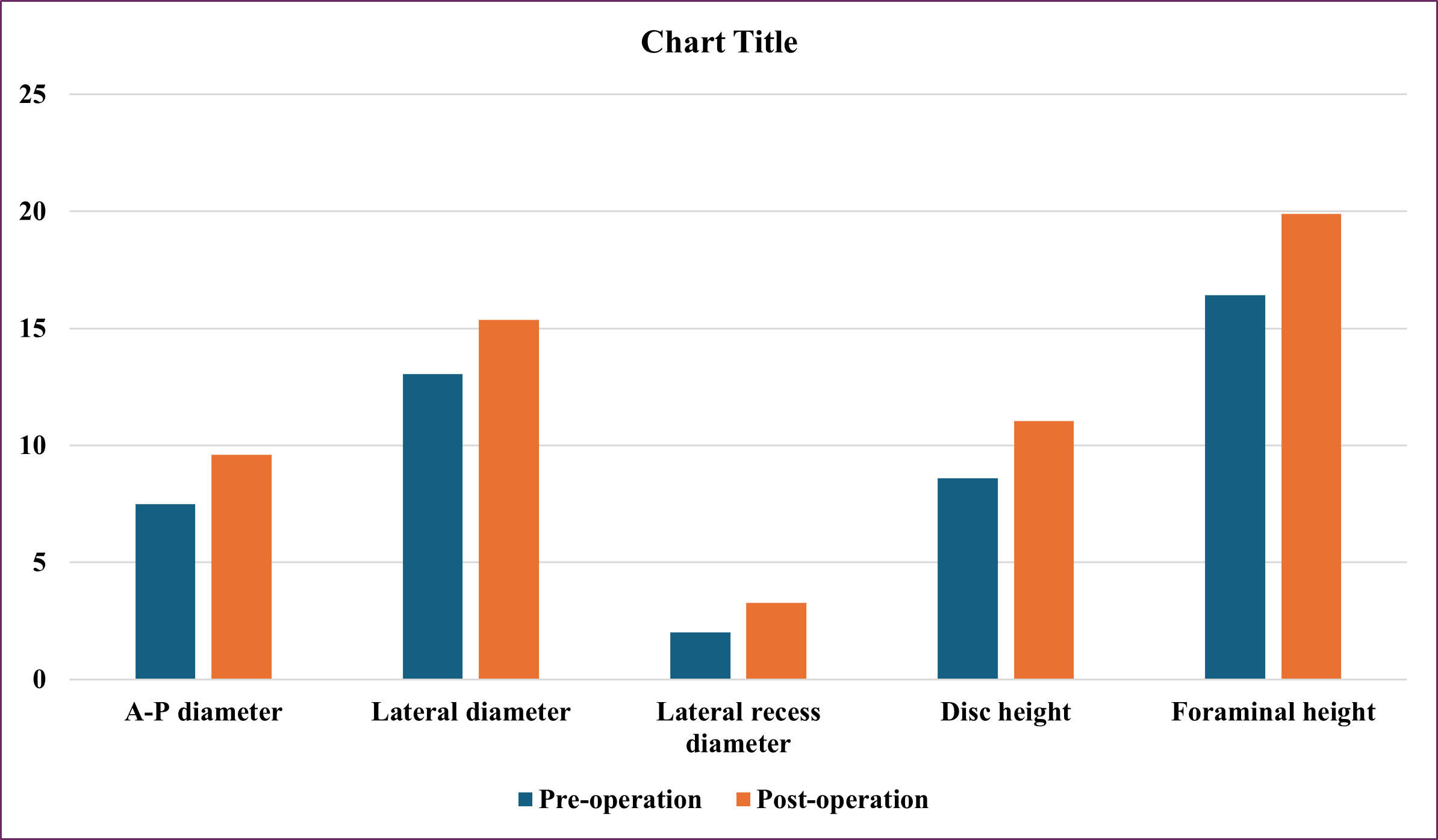
Figure 2: Change MRI in Spinal Canal Area After Surgery
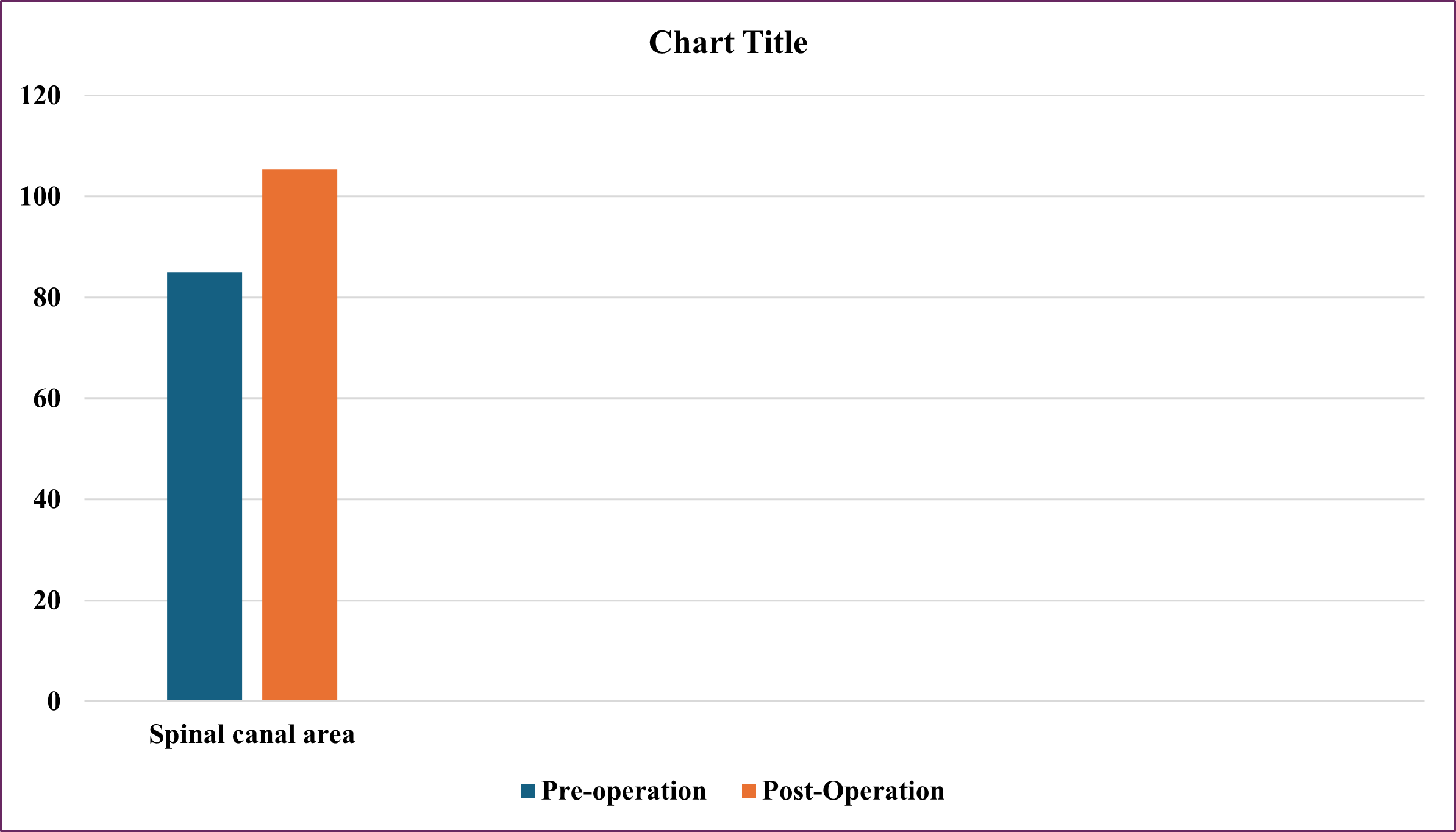
Figure 3: Segmental Lordosis and Lumbar Lordosis Pre- and Post-Operation
Discussion
Indirect decompression through eXtreme Lateral Lumbar Interbody Fusion has been shown to achieve similar or better outcomes with regard to pain and disability relief compared to direct approaches [4].
In our research group, there were 29 cases of lumbar spinal stenosis and 4 cases of lumbar spondylolisthesis (Table 1). The main clinical symptoms are back pain accounting for 97%, radiculopathy accounting for 81.8% and neurogenic claudication 84.8% (Table 1).
Table 1: Demographic Data
|
Variable |
Value |
|
Sex |
|
|
Male |
14(42.4) |
|
Female |
19(57.6) |
|
Age (years) |
57.58±8.573(36-74) |
|
Lumbar stenosis |
29 (87.9) |
|
Lumbar spondylolisthesis |
4 (12.1) |
|
Segments of XLIF 1-segment 2-segment |
30 (90.9) 3 (9.1) |
|
Level distribution (n=36) L23
L34 L45 |
1 (2.8) |
|
6 (16.7) |
|
|
29(80.6) |
|
|
Blood loss (ml) |
42.88±85.305 (10-500) |
|
Time surgery (minutes) |
125.45±37.089 (90-210) |
|
Length of hospital stay (days) |
6.09±2.765 (2-14) |
|
Time follow-up (months) |
17.51 (1–62) |
|
Clinical sign Lumbar back pain Radiculopathy Neurogenic claudication |
97% 81.8% 84.8% |
|
Complications |
|
|
Pedicle screw malformation |
1 (3) |
ALL avulsions fracture. |
1 (3) |
|
Abdominal herniation |
1 (3) |
|
Venous damage |
1(3) |
|
Failure |
1 (3) |
Fusion results by Bridwell grading (n=14)Grade 1 |
6 (42.9) 8 (57.1) |
Values are presented as a number (%) or mean (range)
ALL, Anterior longitudinal ligament.
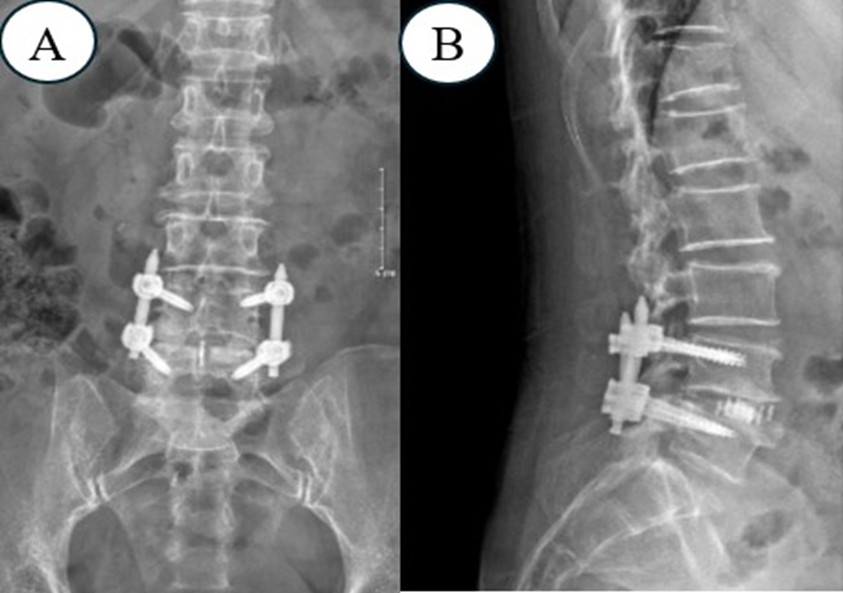
Figure 4. X-Ray After Surgery to Evaluate the Accuracy of Screws and Cage
There were 30 cases of 1-segment XLIF surgery, and 3 cases of 2-segment XLIF surgery (Table 1). The average surgery time was 125.45±37.089 minutes, the average blood loss was 42.88±85.305 ml, and there was 1 case of 500ml blood loss due to damage to the iliac vein. There were no cases requiring blood transfusion during or after surgery, the average hospital stay was 6.09±2.765 days (Table 1). There was 1 segment at L23, 6 segments at L34, and 29 segments at L45.
Assessing the VAS score after surgery, the back VAS score decreased from 7.19±1.75 to 2.63±1.66 after surgery (P<0.001) (Table 2). The leg VAS decreased from 6.75±2.09 to 1.19±1.77 after surgery (p<0.001) (Table 2). Rogers et al[5] studied XLIF surgery for 63 patients with grade II spondylolisthesis, with an average follow-up period of 12 months. The results showed that the most common surgical level was L4-5 (97%), 84% of patients were female, and the average age was 66. The majority of patients (71%) had undergone previous lumbar spine surgery. The average amount of blood loss decreased by 1.4g (after surgery compared to before surgery), and the average hospital stay was 1.2 days. 2 cases (3.4%) of complications were: 1 case of intestinal obstruction after surgery, 1 case of screw fracture 14 months after a traffic accident. There was no nerve damage or infection. VAS score improved by 75% (8.7 to 2.2), disc height increased by 96% (4.6mm to 9.0mm), and slippage improvement was 11.1mm to 3.6mm. Most patients had complete bone union with an improved Lenke score of 1.1 after 12 months. 89% of patients described being satisfied or very satisfied with the results.
X-ray examination after surgery showed that 1 case had pedicle screw malformation. The patient showed signs of nerve root compression. Postoperative X-ray showed pedicle screw malformation. After 2 days, the patient had surgery to reset the screw, and all in cases the cage was placed in the correct position (Table 2).
There was one case (3%) of failure after indirect decompression surgery, however, after direct posterior decompression surgery, there was no compression and after a period of rehabilitation, the patient recovered well. Oliveira et al[6] reported that 9.5% of patients had insufficient relief of nerve compression symptoms and required additional direct posterior decompression. The causes of failure included cage subsidence, loss of sagittal alignment correction, and persistent central and foraminal stenosis.
Rentenberger et al [7] reported an 18.8% reoperation rate due to neurological symptoms, pain, or radiculopathy. Kim et al [8] showed that the rate of additional posterior decompression after XLIF was 60% while Park et al [9] reported that the rate of posterior decompression after indirect decompression and instrumentation was as high as 72.1% in patients with leg pain that improved ≤ 50% after the index procedure. A few reports have provided clear guidance for selecting appropriate patients for indirect decompression. Lim et al [10] proposed the prerequisite of preoperative postural pain status to guide patient selection for indirect decompression with XLIF. The ability to achieve a pain-free position, such as sitting or lying, was a clinical predictor of successful XLIF for patients with lumbar spinal stenosis. Gabel et al [11] suggested an algorithmic approach to predict the success of indirect decompression with LLIF. Patients who achieved pain relief at rest and locked facet fusion, free disc fragments, facet cysts, osteoporosis, and severe spinal stenosis were unlikely to require revision surgery for direct decompression. In the study by Wicharn Yingsakmongkol and colleagues in 2022 [12], the success rate of indirect lateral decompression surgery was 93.3%. The author also commented that patient selection for surgery plays an important role in the success of the surgery. The author selected patients with pain relief when walking, standing, and resting. The height of the intervertebral disc increased by at least 1mm when in a lying position, with no muscle weakness greater than grade IV, no posterior compression such as a cyst, or joint facet, and no migrated disc herniation. Do not select patients with congenital spinal stenosis or short spinal pedicles, do not select patients with bone spurs compressing the lateral recess, and do not select patients with radicular pain without Improved bending position. The author also commented that during surgery, a disc height of at least 10mm will increase the likelihood of success. In the study of Sertac Kirnaz et al [13], they showed that one of the causes of failure of decompression is bony lateral recess stenosis. The author also suggests direct decompression for severe cases of lateral recess stenosis.
Timothy Y. Wang et al [14] also concluded that bony lateral recess stenosis is an important factor of failure in indirect decompression surgery. Of 45 patients (age 65.6 ± 10.5 years; 14 male) involving 101 spinal levels included in this study, 13 (29%) failed indirect decompression.
There was 1 case (3%) of abdominal wall hernia after surgery requiring abdominal wall restoration surgery (Table 2), 1 case of ALL avulsion fracture, and 1 case of pedicle screw malformation which required reoperation to adjust the pedicle screw. There were no cases of dural damage, major blood vessel damage, or post-operative infection.
Table 2: Summary of Clinical Outcomes
|
Variable (n=33) |
Preoperative |
Postoperative |
P-value
|
|
VAS for back pain |
7.19±1.75 |
2.63±1.66 |
<0.001 |
|
VAS for leg pain |
6.75±2.09 |
1.19±1.77 |
<0.001 |
|
ODI |
26.78±9.29 |
14.09±8.56 |
<0.001 |
|
JOA score |
7.38±2.95 |
13.75±2.06 |
<0.001 |
In our research group, 27 segments of surgery floors had postoperative magnetic resonance imaging to evaluate the size of the spinal canal, disc height, lateral recess depth, and foraminal height after surgery (Figure 5). In those 27 segments of surgery, the anterior and posterior diameter increased from 128% of pre-operation, the lateral diameter increased from 117% of pre-operation, the lateral recess depth increased from 156% of pre-operation, the spinal canal area increased from 124% of pre-operation, disc height increased from 128% of pre-operation, foraminal height increased 121% of pre-operation (Figure 1 and 2). All differences are statistically significant with p<0.001. In Hiroaki Nakashima’s study, the thecal sac increased by 189% [15].
In Wicharn Yingsakmongkol's study [12], it was shown that in the successful group the disc height increased from 8.08 mm to 12.195 mm, in the failed patient group the disc height increased from 7.47 mm up to 9.39 mm, foraminal height in the success group increased from 17.05 mm to 19.7 mm, in the failed group increased from 16.58 mm to 18 mm.
Figure 5: MRI Before (A) and After Surgery (B)
Not restoring adequate lumbar lordosis during lumbar fusion surgery may result in mechanical low back pain, sagittal unbalance, and adjacent segment degeneration [16]. In our study, segmental lordosis increased from 2.95±4.54 to 7.35±3.42, lumbar lordosis increased from 27.97±14.61 to 32.05±12.52 (Figure 3). According to Kepler CK et [17] the mean preoperative segmental lordosis was 4.1° at the surgical level compared with 7.8° postoperatively (P < 0.01). The average increase is therefore 3.7° per level. The average preoperative lordosis was 1.6° at L1–2, 3.8° at L2–3, 4.8° at L3–4, and 4.3° at L4–5. Mean postoperative lordosis was 6° at L1–2, 6.6° at L2–3, 7.9° at L3–4, and 10° at L4–5. The increase in lumbar lordosis at each level was significantly different (P < 0.05). There was no statistically significant difference in the degree of lordosis increase between levels (P > 0.05 for all differences). The lumbar lordosis increased the most when the cage was placed in the anterior part of the disc space (+7.4° curvature at each level) and less when it was placed in the middle part of the disc (+3.8° curvature per level). Once it is in the posterior part of the disc space, actual kyphosis is created (-1.2° of curvature per level); these differences are statistically significant (P = 0.017). Analysis of cage tilt did not show that cage tilt had no effect on postoperative spinal lordosis, regardless of whether the data were analyzed using two groups (tilt <5° and tilt > 5°, P > 0.1) or three groups (tilt <5°, tilt 5°–10°, and tilt > 10°, P > 0.2). The height of the cage also did not affect the degree of spinal curvature after surgery (P > 0.2). Analysis of the incidence of postoperative neurological symptoms (sensory or motor) showed no difference between cage placement in the anterior/middle of the disc (rate = 22%) or the posterior part of the disc (rate = 33%, P = 0.62) Mean total preoperative lumbar lordosis was 43.5° compared with 48.4° postoperatively (P = 0.14) because of increased 3° per level. The mean preoperative sacral slope was 32.5° compared with 34.5° postoperatively (P = 0.19) an increase of 2° per level.
Age was not significantly correlated with change in partial or total lordosis (r = 0.09, P = 0.23, r= 0.12, P = 0.27, respectively). Gender was not significantly associated with increased segmental or total lordosis (P = 0.8, P = 0.9, respectively); Neither did BMI (r = -0.1, P = 0.24, r = 0.13, P = 0.25, respectively).
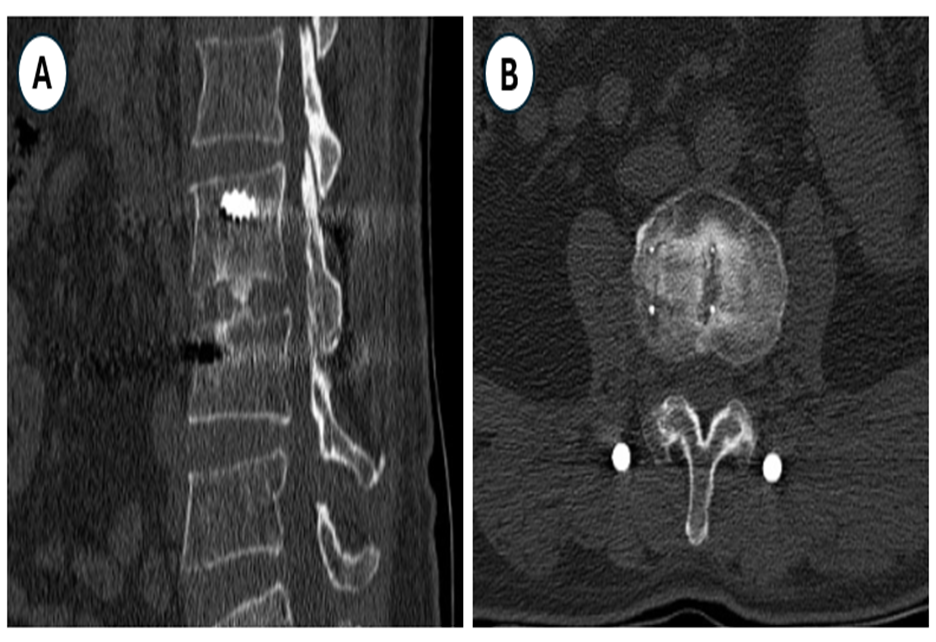
In our study, there were 14 surgical stages that were followed for more than 6 months, with X-rays or CT scans, showing that the bone fusion rate of grade 1 was 42.9%, and grade 2 was 57.1%. This is also the advantage of XLIF surgery when placing a larger cage. In Kanthika Wasinpongwanich's study, comparing the bone fusion rate of XLIF with TLIF, it showed that after 1 year, the bone fusion g rate of XLIF was better (72.7% compared to 83.07%), however after 2 years, the rate was no different [18].
Figure 6. The Lumbar Spine CT Scanner 6 Months Post-Surgery Evaluated the Bone Fusion Grade (A-B).
Rodgers et al [19] compared the complications of 60 patients aged 80 years or older undergoing interbody fusion (20 PLIF patients and 40 XLIF patients). The average number of PLIF treatment floors was 2.6 while the average number of XLIF treatment floors was 1.6. When comparing, the author commented that the blood loss rates of PLIF and XLIF were 2.7g and 1.4g respectively, blood transfusion rates were 70% and 0%, complications were 60% vs 7.5%, The length of hospital stays was 5.3 days versus 1.3 days, the reoperation rate was 15% versus 5%, and the 6-month mortality rate was 30% versus 2.5%. In a separate study of XLIF, the authors compared patients with BMI below and above 30 (obesity threshold). The author also concluded that there were similarities in hospital stay, blood loss, and complications (the author did not mention any cases of infection in either group). From there, the author concluded that the risk of patients with high age and BMI in traditional surgery had changed in XLIF [20] surgery.
Conclusion
XLIF surgery is a minimally invasive surgery through the psoas muscle. It is effective in indirect decompression for lumbar spinal stenosis both clinical and imaging. This method is also suitable for low-grade lumbar spondylolisthesis. This method reduces blood loss, reduces post-operative pain, and indirectly decompresses and restores lumbar lordosis.
Conflict of Interest
The authors have nothing to disclose.
Funding/Support
This study received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
Author Contribution
Conceptualization or design of the work: HKD, TCN, VN; Data acquisition: TMH; Analysis or interpretation: TMH, Writing or Manuscript Revision: TMH.
- McAfee PC, Regan JJ, Geis WP, Fedder IL. (1998). Minimally invasive anterior retroperitoneal approach to the lumbar spine. Emphasis on the lateral BAK. Spine (Phila Pa 1976). 23(13): 1476–1484.
- Hughes A, Makirov SK, Osadchiy V. (2015). Measuring spinal canal size in lumbar spinal stenosis: description of method and preliminary results. Int J Spine Surg. 9:3.
- Bridwell KH, Lenke LG, McEnery KW, Baldus C, Blanke K. (1995). Anterior fresh frozen structural allografts in the thoracic and lumbar spine. Do they work if combined with posterior fusion and instrumentation in adult patients with kyphosis or anterior column defects? Spine (Phila Pa 1976). 20(12): 1410–1418.
- Rabau O, Navarro-Ramirez R, Aziz M, Teles A, Mengxiao Ge S, Quillo-Olvera J. (2020). Lateral Lumbar Interbody Fusion (LLIF): An Update. Global Spine J. 10(2): 17-21.
- Rodgers WB, Lehmen JA, Gerber EJ, Rodgers JA. (2012). Grade 2 spondylolisthesis at L4-5 treated by XLIF: safety and midterm results in the “worst case scenario”. TheScientificWorldJournal. 356712.
- Oliveira L, Marchi L, Coutinho E, Pimenta L. (2010). A radiographic assessment of the ability of the extreme lateral interbody fusion procedure to indirectly decompress the neural elements. Spine. 35(26): 331–337.
- Rentenberger C, Okano I, Salzmann SN, Winter F, Plais N, Burkhard MD. (2020). Perioperative Risk Factors for Early Revisions in Stand-Alone Lateral Lumbar Interbody Fusion. World Neurosurg. 134: 657–663.
- Kim SJ, Lee YS, Kim YB, Park SW, Hung VT. (2014). Clinical and radiological outcomes of a new cage for direct lateral lumbar interbody fusion. Korean J Spine. 11(3): 145–151.
- Park D, Mummaneni PV, Mehra R, Kwon Y, Kim S, Ruan HB. (2020). Predictors of the need for laminectomy after indirect decompression via initial anterior or lateral lumbar interbody fusion. J Neurosurg Spine. 32(6): 781-787.
- Lim KZ, Daly C, Brown J, Goldschlager T. (2019). Dynamic Posture-Related Preoperative Pain as a Single Clinical Criterion in Patient Selection for Extreme Lateral Interbody Fusion Without Direct Decompression. Global Spine J. 9(6): 575–582.
- Gabel BC, Hoshide R, Taylor W. (2015). An Algorithm to Predict Success of Indirect Decompression Using the Extreme Lateral Lumbar Interbody Fusion Procedure. Cureus. 7(9): 317.
- Yingsakmongkol W, Jitpakdee K, Kerr S, Limthongkul W, Kotheeranurak V, Singhatanadgige W. (2022). Successful Criteria for Indirect Decompression With Lateral Lumbar Interbody Fusion. Neurospine. 19(3): 805–815.
- Kirnaz S, Navarro-Ramirez R, Gu J, Wipplinger C, Hussain I, Adjei J. (2020). Indirect Decompression Failure After Lateral Lumbar Interbody Fusion-Reported Failures and Predictive Factors: Systematic Review. Global Spine J. 10(2): 8-16.
- Wang TY, Nayar G, Brown CR, Pimenta L, Karikari IO, Isaacs RE. (2017). Bony Lateral Recess Stenosis and Other Radiographic Predictors of Failed Indirect Decompression via Extreme Lateral Interbody Fusion: Multi-Institutional Analysis of 101 Consecutive Spinal Levels. World Neurosurg. 106: 819–826.
- Nakashima H, Kanemura T, Satake K, Ishikawa Y, Ouchida J, Segi N. (2019). Indirect Decompression on MRI Chronologically Progresses After Immediate Postlateral Lumbar Interbody Fusion: The Results From a Minimum of 2 Years Follow-Up. Spine (Phila Pa 1976). 44(24): 1411–1418.
- Barrey C, Darnis A. (2015). Current strategies for the restoration of adequate lordosis during lumbar fusion. World J Orthop. 6(1): 117–126.
- Kepler CK, Huang RC, Sharma AK, Meredith DS, Metitiri O, Sama AA. (2012). Factors influencing segmental lumbar lordosis after lateral transpsoas interbody fusion. Orthop Surg. 4(2): 71–75.
- Wasinpongwanich K, Nopsopon T, Pongpirul K. (2022). Surgical Treatments for Lumbar Spine Diseases (TLIF vs. Other Surgical Techniques): A Systematic Review and Meta-Analysis. Front Surg. 9: 829469.
- Rodgers WB, Gerber EJ, Rodgers JA. (2010). Lumbar fusion in octogenarians: the promise of minimally invasive surgery. Spine. 35(26): 355–360.
- Rodgers WB, Cox CS, Gerber EJ. (2010). Early complications of extreme lateral interbody fusion in the obese. Journal of spinal disorders & techniques. 23(6): 393–397.
Download Provisional PDF Here
PDF




p (1).png)




.png)




.png)
